s h2so4|s h2so4 long : Cebu S + H2SO4 = SO2 + H2O is a Double Displacement (Metathesis) reaction where one mole of Sulfur [S] and two moles of Sulfuric Acid [H 2 SO 4] react to form three moles of Sulfur Dioxide [SO 2] and two moles of Water [H 2 O] O “quiero agua vã deo gore viral video” é uma experiência visual e emocional. Gomez projetou essa peça com a intenção de fomentar a reflexão sobre a sociedade atual e suas estruturas. O vídeo é uma representação metafórica de como as pessoas muitas vezes anseiam por algo que não podem obter ou que está além de seu alcance.
0 · what is the oxidation state of s in h2so4 & h2so3
1 · s h2so4 long
2 · s h2so4 balanced equation
3 · h2so4chemical name
4 · h2so4 to h2s half equation
5 · h2so4 s oxidation number
6 · h2so4 equation formula
7 · h2so4 compound name
8 · More
Acompanhe as notícias do São Paulo no ge.globo, tudo sobr.
s h2so4*******S + H2SO4 = SO2 + H2O is a Double Displacement (Metathesis) reaction where one mole of Sulfur [S] and two moles of Sulfuric Acid [H 2 SO 4] react to form three moles of Sulfur Dioxide [SO 2] and two moles of Water [H 2 O]S + H2SO4 = SO4 + H2S is a Double Displacement (Metathesis) reaction where one mole of Sulfur [S] and one mole of Sulfuric Acid [H 2 SO 4] react to form one mole of Sulfur .s h2so4 longSulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water. Pure sulfuric acid does not occur naturally due to its strong affinity to water vapor; it is hygroscopic and .
A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in . In the $\ce{H2SO4}$ molecule, two bonds are simple covalent ($\ce{S-OH}$ ones), and two are dative ($\ce{S-O}$ ones). A .
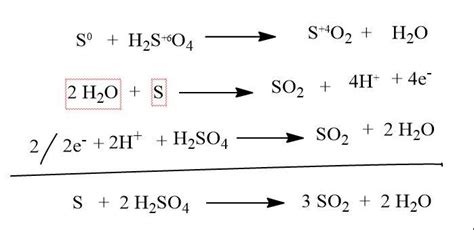
Solution. Step 1: Given equation. S + H 2 SO 4 → SO 2 + H 2 O. Step 2: Method used for balancing the equation. Using the traditional method of balancing chemical equations. .Solved and balanced chemical equation H2SO4 + H2S → SO2 + 2 H2O + S with completed products. Application for completing products and balancing equations.
Sulfuric acid (H2S04) is a corrosive substance, destructive to the skin, eyes, teeth, and lungs. Severe exposure can result in death. Workers may be harmed from exposure to sulfuric acid. The level of exposure . Học tập về phương trình phản ứng S + H2SO4 → SO2 + H2O, đặc nhiệt độ, cách tiến hành, hiện tượng và tính chất của lưu huỳnh. Xem bài tập vận dụng minh họa và tải file VnDoc.com. ACGIH: Documentation of the Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) - Sulfur Dioxide. See annual publication for most recent .
S + HNO3 → H2SO4 + NO2 + H2O | S ra H2SO4 | HNO3 ra NO2 - Hướng dẫn cân bằng phản ứng hóa học của tất cả phương trình hóa học thường gặp giúp bạn học tốt môn Hóa. Nhà sách VietJack. Toggle navigation. Lớp 1. Giải Vở bài tập Tiếng Việt lớp 1 - KNTT;S + H2SO4 = SO2 + H2O - Ecuación química balanceada. Utiliza la calculadora de abajo para equilibrar las ecuaciones químicas y determinar el tipo de reacción (Instrucciones). Balanceador de Ecuaciones Químicas. 🛠️ Balancea la ecuación .S + H2O = H2SO4 + H2S - Phương trình hoá học đã cân bằng. Sử dụng máy tính bên dưới để cân bằng phương trình hóa học và xác định loại phản ứng (hướng dẫn) . Trình cân bằng phản ứng hoá học. 🛠️.

1. H2SO4 + H2S → H2O + S + SO2. Solved and balanced chemical equation H2SO4 + H2S → SO2 + 2 H2O + S with completed products. Application for completing products and balancing equations.Word Equation. Barium + Sulfuric Acid = Barium Sulfate + Dihydrogen. Ba + H2SO4 = BaSO4 + H2 is a Single Displacement (Substitution) reaction where one mole of solid Barium [Ba] and one mole of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of solid Barium Sulfate [BaSO 4] and one mole of Dihydrogen [H 2] gas
Sulfuric acid ( American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling ), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water.
Special H2SO4, nongtac use with copper, sulfur dioxide gases SO2 and blue CuSO4 solution. Reactants. Copper - Cu. Cuprum Element 29 Paragard T 380A Tatum-T Cu-7. Cu Molar Mass Cu Oxidation Number. Sulfuric Acid - H 2 SO 4 [S(Oh)2O2] [So2(Oh)2] Oil Of Vitriol Hydrogen Sulfate Battery Acid Sulphuric Acid H2So4. Pure sulfuric acid has a specific gravity of 1.830 at 25 °C (77 °F); it freezes at 10.37 °C (50.7 °F). When heated, the pure acid partially decomposes into water and sulfur trioxide; the latter escapes as a vapour until the concentration of the acid falls to 98.3 percent. This mixture of sulfuric acid and water boils at a constant .Word Equation. Iron + Sulfuric Acid = Iron (Ii) Sulfate + Dihydrogen. Fe + H2SO4 = FeSO4 + H2 is a Single Displacement (Substitution) reaction where one mole of solid Iron [Fe] and one mole of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Iron (Ii) Sulfate [FeSO 4] and one mole of Dihydrogen [H 2] gas.
Balance the following equation: h) S+H 2SO4 → SO2 +H 2O. 1.) FeCl 3 + SO 2 + H 2 O→ FeCl 2 + HCL + H 2 SO4. 2.) S + HNO 3 → H 2 SO 4 + NO 2 + H 2 O. Balance the following equations by oxidation number method. Balance the following equation by oxidation number method.
H2S + H2SO4 → SO2↑ + H2O + S↓. 2. Điều kiện phản ứng H2S tác dụng H2SO4 đặc. 3. Hiện tượng phản ứng xảy ra. 4. Bài tập vận dụng liên quan. H 2 S + H 2 SO 4 → SO 2 + H 2 O + S được VnDoc biên soạn hướng dẫn các bạn viết và cân bằng phương trình phản ứng giữa H 2 S và H 2 SO .s h2so4Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 3 Zn + 4 H2SO4 = 3 ZnSO4 + S + 4 H2O. Reactants.
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Mn + H2SO4 = MnSO4 + H2. Reactants. Products.s h2so4 s h2so4 longStep 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. S + 6 HNO3 = H2SO4 + 6 NO2 + 2 H2O. Reactants. H2S + H2SO4 → SO2↑ + H2O + S↓. 2. Điều kiện phản ứng H2S tác dụng H2SO4 đặc. 3. Hiện tượng phản ứng xảy ra. 4. Bài tập vận dụng liên quan. H 2 S + H 2 SO 4 → SO 2 + H 2 O + S được VnDoc biên soạn hướng dẫn các bạn viết và cân bằng phương trình phản ứng giữa H 2 S và H 2 SO .Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 3 Zn + 4 H2SO4 = 3 ZnSO4 + S + 4 H2O. Reactants.Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Mn + H2SO4 = MnSO4 + H2. Reactants. Products.Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. S + 6 HNO3 = H2SO4 + 6 NO2 + 2 H2O. Reactants.Word Equation. Sodium Sulfide + Sulfuric Acid = Sodium Sulfate + Hydrogen Sulfide. Na2S + H2SO4 = Na2SO4 + H2S is a Double Displacement (Metathesis) reaction where one mole of aqueous Sodium Sulfide [Na 2 S] and one mole of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Sodium Sulfate [Na 2 SO 4] and one mole of solid .Word Equation. Potassium + Sulfuric Acid = Potassium Sulfate + Dihydrogen. K + H2SO4 = K2SO4 + H2 is a Single Displacement (Substitution) reaction where two moles of solid Potassium [K] and one mole of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Potassium Sulfate [K 2 SO 4] and one mole of Dihydrogen [H 2] gas.Kyselina sírová reaguje se zinkem za vzniku vodíku a síranu zinečnatého. Kyselina sírová reaguje s hliníkem za vzniku vodíku a síranu hlinitého. Podobně většina oxidů kovů se v kyselině sírové rozpouští za vzniku solí. Oxid měďnatý reaguje s kyselinou sírovou za vzniku vody a síranu měďnatého. Reakcí s amoniakem .Aluminium + Sulfuric Acid = Aluminum Sulfate + Dihydrogen. Al + H2SO4 = Al2 (SO4)3 + H2 is a Single Displacement (Substitution) reaction where two moles of solid Aluminium [Al] and three moles of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Aluminum Sulfate [Al 2 (SO 4) 3] and three moles of Dihydrogen [H 2] gas.
Word Equation. Sulfur + Dioxygen + Water = Sulfuric Acid. S + O2 + H2O = H2SO4 is a Synthesis reaction where two moles of Sulfur [S], three moles of Dioxygen [O 2] and two moles of Water [H 2 O] combine to form two moles of Sulfuric Acid [H 2 SO 4] Show Chemical Structure Image.
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Mg + H2SO4 = MgSO4 + H2. Reactants. Products.
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. CuS + 4 H2SO4 = CuSO4 + 4 SO2 + 4 H2O. Reactants.
CuO (s) + H 2 SO 4 (aq) → CuSO 4 (aq) + H 2 O (l) Kyselina sírová sa tiež používa na vytesnenie slabších kyselín z ich solí, napríklad kyseliny octovej z octanu sodného : H 2 SO 4 + CH 3 COONa → NaHSO 4 + CH 3 COOH. Podobne môže byť pripravená kyselina dusičná z dusičnanu draselného. Ako druhý produkt vzniká .
webAssistance. For assistance with UNBC Online Services please direct your inquiry to one of the following: Account and personal information. Students – contact the Office of the .
s h2so4|s h2so4 long